Since the Valsartan scandal in June 2018, there has been growing interest in the risk assessment of potential traces of toxic nitrosamines in drug substances and products. The Official Medicines Control Laboratories (OMCLs) of the General European OMCL Network (GEON) were involved in investigations and actions to address the issues related to the detection of N-nitrosodimethylamine (NDMA), N-nitrosodiethylamine (NDEA) and other concerned nitrosamines. The EMA gives the manufacturers six month for risk based testing and three years for all the necessary tests and to implement corresponding changes in the manufacturing license.
Based on a CVUA Karlsruhe method DSI-pharm now implemented a generic LC-MS/MS method with limits of quantification (LOQ) between 1 ng/mL and 5 ng/mL for 6 different nitrosamines including NDMA and NDEA (current spectrum of nitrosamines contained see Table and Figure 1). Samples of drug substances and products are tested for nitrosamine traces after dilution in solvent by liquid chromatography coupled with tandem mass spectrometry using atmospheric pressure chemical ionization (UHPLC-APCI-MS/MS) in multiple reaction monitoring mode (MRM).
The use of LC-MS/MS technology for the testing of nitrosamine traces allows for a quick and effective sample solvent dilution with high recovery.
The generic LC-MS/MS method can be easily adapted to different drug substances and products if necessary. The method must then be validated under GMP for each product. The final LOQ´s will range between about 0,010 ppm and 0,050 ppm depending on the nitrosamines and the required sample dilution. Upon request, further nitrosamines of interest can be implemented into the method.
DSI-pharm is highly experienced in trace residue testing in food and pharma products and can offer you attractive turnaround times – also for method development and validation.
Table 1: Nitrosamines included in method
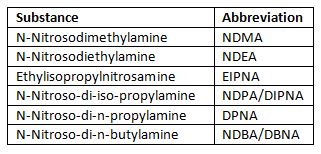
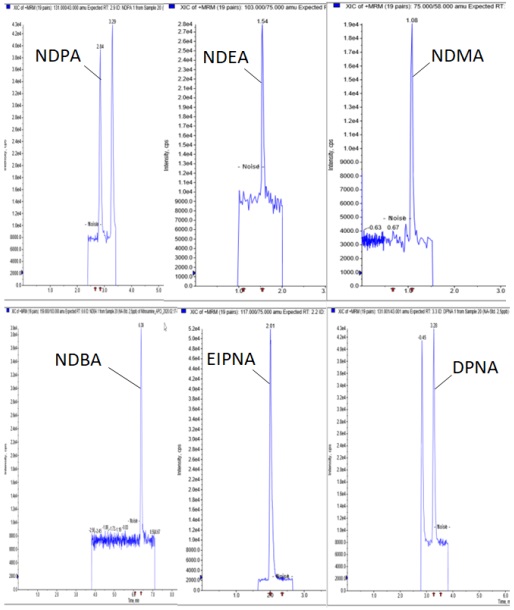
Figure 1: Nitrosamines, exemplary Chromatograms at LOQ level (1 ng/mL to 5 ng/mL)
Do you have further questions about analysis of nitrosamines?
Please do not hesitate to contact Dr. Serap Acikgöz:
Email: analytics.bre@tentamus.com
Tel: +49 (0)421 / 59 66 070
Bremen, 04.03.2020 

